
We’ve all heard the term Greenhouse Effect, but have you ever wondered what it truly means? Michael Konrad of South Forty Pier sends along the following explanation of this climate phenomenon — and much more.
Climate change caused by global warming is frequently in the news. The floating home community is interested — 26 articles on the subject have been published in the Floating Times since 2017. This is hardly surprising since higher sea levels and more intense rainstorms are examples of climate change, and already the entrances to Kappas piers and the Yellow Ferry parking areas occasionally flood to levels that make access difficult.
However, the subject has become politically and culturally controversial, if not divisive, with those who believe rising CO2 levels are the result of human activity, and through the greenhouse effect, are the main cause of climate change, being labeled “climate alarmists”, and those who don’t believe this being labeled “climate deniers”. A search in the Amazon.com book section finds almost half of the books for sale there obviously written by (or perhaps for) climate deniers. Most commentators on Fox News, a major cable channel, are climate deniers, as is Donald Trump, the President of the United States, who calls it “fake news”. His entire administration, the ones that talk about it anyway, shares this viewpoint, although us biologists realize this is likely just an example of Darwin’s principle of selection, the “survival of the deniers”.
This article does not discuss the broad subject of climate change and what we should do about it, if anything. However, in the spirit of full disclosure I declare that I am a “climate alarmist”. I thus throw my lot in with the IPCC (the Intergovernmental Panel on Climate Change, more than 200 international scientists in the field), 98 percent of American scientists, scientific journals, Wikipedia, the BBC, the New York Times, etc. Controversy is avoided in this article by only presenting established scientific facts about the greenhouse effect itself. Both alarmists and deniers will want a solid understanding of the greenhouse effect in order to generate more informed and thus persuasive arguments. Surprisingly we will find that the physics needed to understand the greenhouse effect is entangled with some of the most important and fundamental scientific discoveries of the 19th and 20th centuries.
Temperature
Temperature is a measure of the kinetic (resulting from motion) energy of molecules and is reported as degrees. The United States uses a version of the British Imperial system of units because originally, many of our founding fathers came from Britain. The British are damn well not going to use the rational metric system invented by the French and now used by the vast majority of the world. However, even the BBC has reported temperature in Celsius, C, since the 1980s. Water freezes at 0 C, boils at 100 C and “room” temperature is 20 C. Here I will use a slight variation of Celsius, the Kelvin or absolute temperature scale. A Kelvin degree is equal to a Celsius degree, but zero K is -273 C, the temperature at which thermal energy is zero. Most physical phenomena are thus most easily described in terms of degrees K.
The greenhouse effect: lite version
The earth’s temperature is determined by the balance between energy coming in from the sun, and energy radiating out into space. For at least the last two hundred years the radiance of the sun, the energy it radiates, has been almost constant. Atmospheric clouds and fog reflect sunlight, as do snow and ice on the earth’s surface, which thus decreases energy input. However, the effects of snow, ice, and clouds average out over a full year and thus do not change the yearly input. The interior of the earth is hot, but the crust provides good insulation, and that source of energy is constant and small so is neglected here.
The rate energy leaves the earth as infrared radiation is very dependent the earth’s temperature. If temperature increases the radiation increases and the earth cools. If temperature decreases, the radiation decreases, and the temperature rises. The effect on this radiation by gases in the atmosphere is not obvious to us because we cannot see infrared radiation. Water, carbon dioxide, methane and other complex molecules all absorb infrared radiation, so if our eyes were sensitive to infrared a visually clear sky containing water vapor, carbon dioxide, or methane would appear gray or black, and we might not be surprised that a resulting blanket effect would increase the temperature. These gases are all called greenhouse gasses because they mimic the effect of glass in a greenhouse, which lets visible sunlight in but bocks most infrared radiation from leaving the warmed greenhouse.
The greenhouse effect: Planck black body distribution and more
Any object with a temperature above 0 K, absolute zero, radiates energy. However, if an object is black, it doesn’t reflect energy, that’s the meaning of “black”. It is easier to calculate the amount of energy radiating from a body if it doesn’t also reflect. Thus, in discussions of the effect of temperature on radiation, the radiation is often called “black body radiation”. However, in most cases, and in our case, it doesn’t matter much that the object isn’t black, and I ignore it here.
A hot object radiates a spectrum, variable intensities over a range of wavelengths, in the form of electromagnetic (EM) energy, e.g. heat, light. The shape of this spectrum is described by the Planck black body distribution which depends only on the temperature. This equation was used to plot the two curves of intensity versus wavelength, seen in Figure 1, for radiation produced by the sun and the earth.
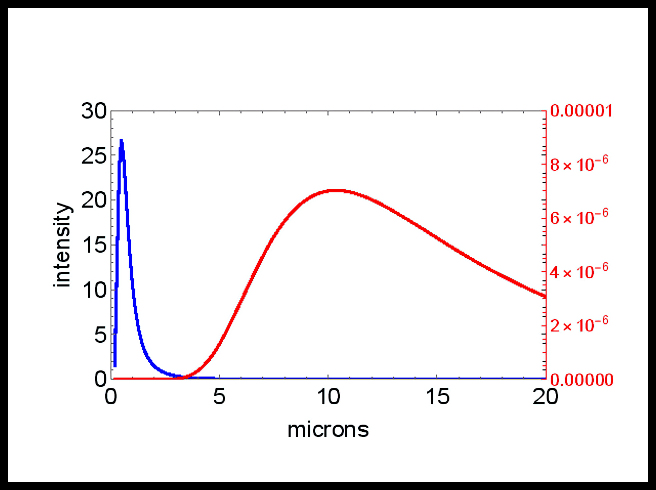
The temperature of the surface of the sun is about 6000 K. Thus, it emits EM, as light, with a peak wavelength of 0.5 µ (microns, or millionths of a meter, or thousandths of a millimeter). This radiation travels through space for about 150 million kilometers and is absorbed at the surface of the earth. The earth’s surface is at a temperature of only 300 K, and thus the energy absorbed by the earth “redshifts”, and radiates back into space with an average wavelength of 12 µ, the far infrared, which is not visible. Redshift is used colloquially to mean any shift to a longer wavelength, because red has the longest wavelength of the visible colors.
Several components of the atmosphere which do not absorb sunlight do however absorb infrared radiation. Examples are water vapor, H2O, carbon dioxide, CO2, and methane, CH3. Infrared radiation causes the atoms in these molecules to stretch, rotate, and bend the chemical bonds that hold them together, which results in absorption at many infrared wavelengths. The major components of the atmosphere, nitrogen, N2 and oxygen, O2 do not interact with visible or infrared radiation because they vibrate only when exposed to much shorter wavelengths. They are thus transparent to both.
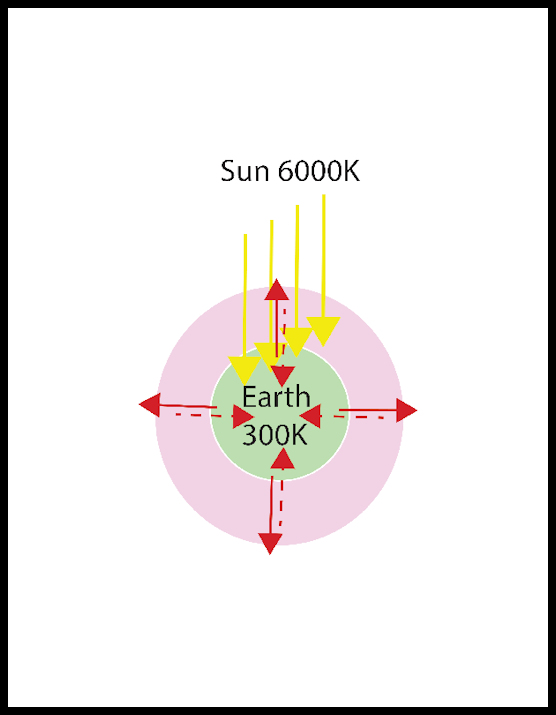
We could imagine EM leaving the sun as a braid of energy which interacts with the earth to radiate out into space with a twenty-fold longer wavelength. Since these radiations have wavelengths, they must be waves, at least in some sense. We will see that this seemingly obvious assumption leads us to some serious problems but persisting we will arrive at an entirely new and profound understanding of space, time and energy.
Maxwell’s electromagnetic waves
By 1860 scientists had done many different electrical and magnetic experiments. Early studies used static electricity to make sparks and shock animals, entertaining guests at parties. When batteries were developed to produce steady direct current, you could measure magnetic fields produced when it flowed through copper wires. Conversely it was found that a changing, but not constant, magnetic field “induced” a current in the same copper wires. Electricity and magnetism must be intimately related in some rather complicated way.
This complicated way became clear when James Clark Maxwell published a set of equations in 1864 that summarized the results of these electrical and magnetic experiments. But surprisingly these four simple equations (four in later improved notations, and simple if you understand the language of vector calculus) also predicted the existence of a traveling wave that had both electrical and magnetic components, the EM we described earlier. Surprisingly the speed of this wave could be calculated from values that had already been determined. It was the speed of light, 3×108 m/sec, already known with sufficient precision for a random coincidence to be quite unlikely. So, light must be just an example of EM, which regardless of wavelength, travels at the same speed, called “c”. Speed, wavelength, and frequency are related by this simple formula:
1] c (m/sec) = wavelength (m) X frequency (cycles per second)
Just 23 years after Maxwell’s publication Heinrich Hertz used spark gaps to both make and detect this EM. Asked about the applications of his discoveries, Hertz replied: “Nothing, I guess.” Today EM over a huge range of wavelengths make much of our life possible. Electrical currents oscillating at a frequency of 60 cycles/sec send EM, with a wavelength of 5,000 km, out from the humming transformers and high voltage power lines that power our world. TV waves are EM with a wavelength of a few m, cell phone signals are EM with lengths of a few cm, we have given the wavelengths of infrared and visible EM, 10 and 0.5 µm. Cobalt 60 emits EM, called gamma rays, with a wavelength of only 0.000001 µ, that destroys tumor cells deep in the body and penetrates steel pipes to verify weld quality of joints. I could go on and on.
This is all fine and good, but Houston, we have a problem. Light and other forms of EM travel very well, in fact best, through a vacuum. If light is a wave, what is it a wave of? Sound is pressure waves in air, water waves are ripples in a water-air interface, earthquake waves are either pressure or lateral waves of the earth. What is the medium for EM? Common sense demands a medium: give it a name, the “ether”, and find it. However, a name didn’t seem to help, as years of many clever, painstaking experiments failed to detect an ether. These experiments all depended on the obvious assumption that light would have different velocities when the observer was moving through the ether at different directions and speeds. But it didn’t. There was no experimental evidence for ether. Light always had the same speed, c, full stop.
Einstein’s relativity
In the early 1900’s Albert Einstein, who had just obtained his degree in physics and loved to do experiments in the laboratory, couldn’t get a job as a professor or even instructor in a university. As a hopefully temporary alternative he got a job as examiner in the Swiss patent office. While he found the work boring, by applying himself he could finish it in the morning and spend most of the afternoon thinking about physics and puzzles being discussed in the scientific literature. He didn’t have access to a laboratory, but he became expert in doing “thought experiments”; if so-and-so were true, what would be the consequences. In our light puzzle, if a pulse of light appeared to move at the same speed relative to you no matter how “fast” you were moving, what other observations would that imply, or require. I put “fast” in quotes, to indicate you need to first describe exactly how you would determine how fast you were moving. Einstein gradually saw what his imagined world would be like, and it was strange. First, if you were moving in a straight line and constant speed you couldn’t tell how fast you were moving if you couldn’t see an “outside” world. Only if you were accelerating could you directly detect movement. Clocks, actually time itself, would not progress at the same rate at high as at low speeds. Meter sticks would increase their length with speed. The mass, m, of an object would not be constant, but would depend on its kinetic energy, e, by the simple formula:
2] e = m c2
A small change in mass would be accompanied by an immense change in energy, since c2 is 9 x 1016, that’s a nine followed by 16 zeros! But the reward for all this strangeness would be that everything was consistent, and light always appeared to have the same speed. He described the world that followed these “principles of relativity” in a 1905 publication, one of his several that year which would revolutionize physics and our understanding of the world we live in.
Fission of uranium
In 1938 Otto Hahn and Fritz Strassmann found that uranium bombarded with neutrons produced barium as one of the products. But the uranium nucleus has a total of 235 neutrons plus protons, while the barium nucleus contains only 137. Many examples of radioactive isotopes had shown that unstable nuclei released relatively small helium nuclei (alpha particles) or gamma rays. However, this was the first time an unstable nucleus appeared to break into two or more large fragments.
Barely a year later during Christmas holidays in 1938, the physicist Lise Meitner, went on a walk and ski trek in the snow with her scientist nephew Otto Frisch. They had been discussing the fragmentation of the uranium nucleus in Hahn’s experiments, as Lise had been a longtime collaborator of Hahn’s, and had complete faith in his identification of barium as one of the products. She used a scrap of paper to calculate the large kinetic energy, 200 MeV, the fragments would acquire due to repulsion between their negative charges, and acceleration to high speeds, and concluded they would radiate this energy as gamma rays. She remembered the factor mc2 from Einstein’s paper and calculated on the spot that this radiating “energy-mass” could make up for the smaller masses of the products of the reaction. It all added up. They quickly published their ideas as a letter to the editor in the 1939, February 11th issue of the journal Nature.
It was obvious that nuclear fission had a potential to be the basis for a bomb, and that, by that time Hitler’s scientists might be working on it. In response, the English started the Top Secret Tube Alloys project. However, only the United States had the necessary economic, industrial, and scientific resources to develop the bomb in the Manhattan Project, which at its end was consuming about 5% of its total GNP. The US also had by that time many extremely competent scientists and engineers, ironically supplemented with most of the brightest minds from Europe who had been expelled by or fled from the Nazi regime. Just after sunrise on the morning of July 16th, 1945, at the Trinity test site, New Mexico, the nuclei of several hundred grams of plutonium fragmented after neutron bombardment, releasing energy equivalent to 20,000 tons of TNT. An intense pulse of gamma rays redshifted to blinding white light and then to infrared radiation, melting the steel tower holding the bomb, the sand under it, and heating the surrounding air to produce a massive pressure wave that lifted tons of sand high into the stratosphere and spread out over the desert.
Equations have consequences.
References
This article presents facts not easily obtained in any one reference. Good places to start are the following entries in Wikipedia, which I used to verify and supplement my memory. All articles in Wikipedia contain extensive references, which make it possible to explore the subjects further.
Black-body radiation, Climate change, Fission, Greenhouse effect, Lise Meitner, Manhattan project, Maxwell’s equations, Special relativity, Thermal radiation, Visible spectrum
I have found the following book a good place to start (or finish) study of climate change: Introduction to modern climate change by Andrew Dessler (2024); Cambridge University Press.